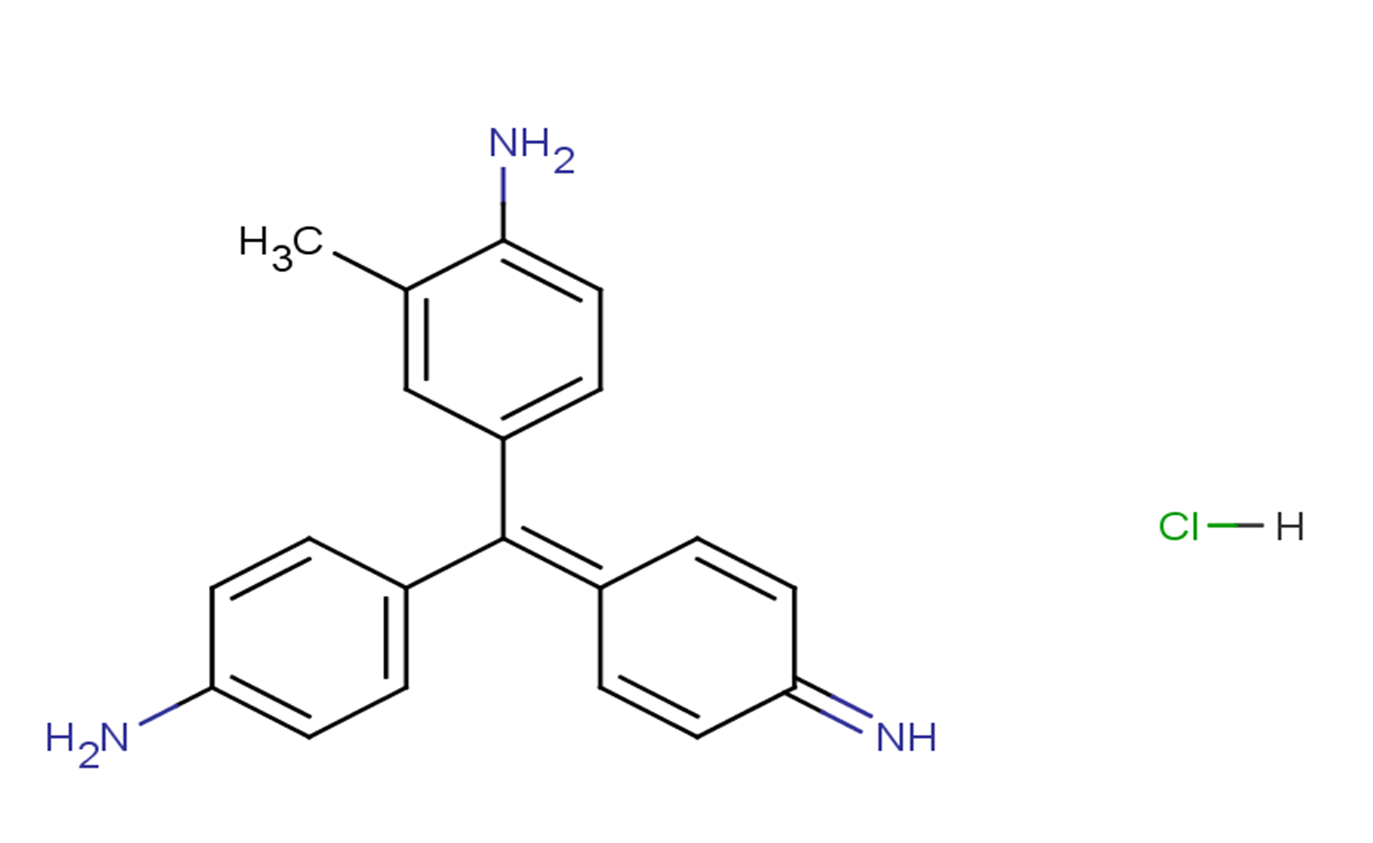
Fuchsine base monohydrochloride
CAS No. 632-99-5
Fuchsine base monohydrochloride( Magenta base monohydrochloride | Basic Fuchsin monohydrochloride | Rosaniline Base monohydrochloride )
Catalog No. M24639 CAS No. 632-99-5
Fuchsine base monohydrochloride is a magenta dye and uses for acid-fast staining with carbol-fuchsin.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameFuchsine base monohydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionFuchsine base monohydrochloride is a magenta dye and uses for acid-fast staining with carbol-fuchsin.
-
DescriptionFuchsine base monohydrochloride is a magenta dye and uses for acid-fast staining with carbol-fuchsin.
-
In Vitro——
-
In Vivo——
-
SynonymsMagenta base monohydrochloride | Basic Fuchsin monohydrochloride | Rosaniline Base monohydrochloride
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number632-99-5
-
Formula Weight337.85
-
Molecular FormulaC20H20ClN3
-
Purity>98% (HPLC)
-
SolubilityDMSO:75 mg/mL (221.99 mM)
-
SMILESNC1=CC=C(/C(C2=CC=C(N)C=C2)=C3C=CC(C=C\3)=N)C=C1C.[H]Cl
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Deacetyltaxol
Extracted from Taxus brevifolia barks.
-
AC-PHE-OME
AC-PHE-OME (Methyl N-acetyl-L-phenylalaninate) is a marine derived natural products found in Family Jaspidae.
-
Glucocamelinin
The herbs of Cardamine leucantha.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


